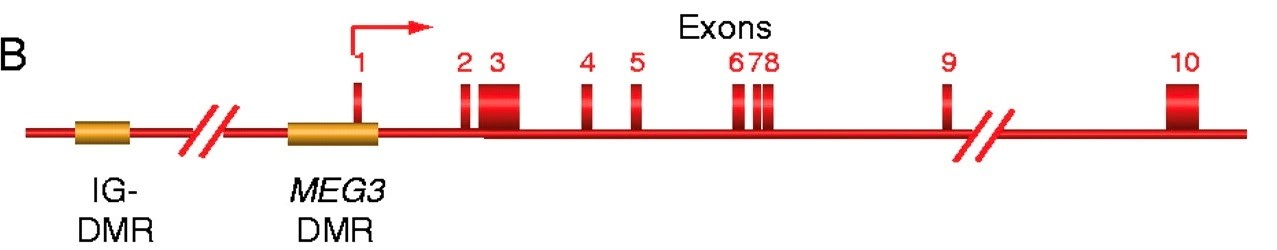
In the United States, there are over 200,000 human pituitary adenomas diagnosed each year. These tumors are the most common form of intracranial neoplasm, and more than 40% of them can be classified as clinically non-functioning adenomas (NFA), which ultimately produce mass neurological dysfunction and hypopituitarism. There is no existing medical therapy to treat these tumors, as the specific underlying cause that produces NFAs is still unknown. In previous research, epigenetic mutations (including both hypermethylation as well as histone modification) have been identified in a wide variety of genes found to be underexpressed or even lost in NFAs. More specifically, MGH’s Neuroendocrine Lab has identified a particular imprinted gene strongly implicated in NFAs: Maternally Expressed Gene 3 (MEG3). This discovery was made through the comparison of gene expression profiles between NFAs and normal human pituitaries, and in later studies MEG3 was identified as a long noncoding RNA tumor suppressor found in the pituitary. While further research also suggested that specific hypermethylation in the promoter and enhancer region of this gene leads to its loss of expression, the mechanisms explaining how this loss of expression contributes to NFA development are unknown. We are now working to understand just that, by exploring MEG3’s tumor suppressor and p53 activation functions through the use of transgenic and knockout murine models. This work will ultimately allow for the development of novel treatment strategies for NFAs and potentially all pituitary adenomas, by allowing for a better understanding of the mechanisms that form them in the first place.