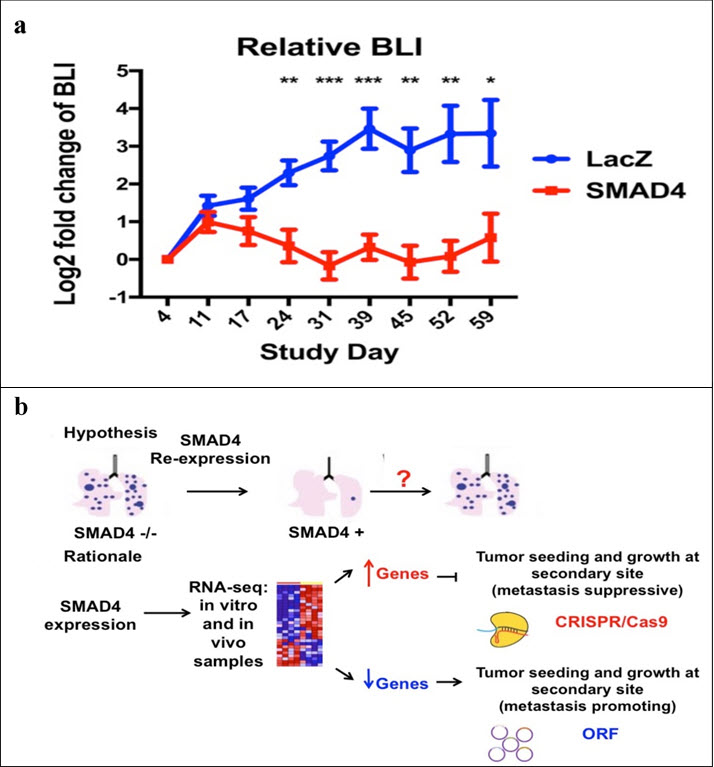
Pancreatic ductal adenocarcinomas (PDA) are a common but deadly form of cancer, with long-term survival made unlikely by the high rate of metastasis found in these tumors. At the time of diagnosis, a vast majority of PDA cases can no longer be sufficiently eliminated by surgery, and thus targeted nanoparticle therapies are a promising route of treatment. Identification of and drug delivery to highly metastatic cells via nanoparticles could greatly aid the long-term prognosis of patients, however first we have to understand what to target. The progression of PDA is often dependent upon the oncogenic activation of KRAS as well as the loss of the tumor suppressors CDKN2A, TP53, and SMAD4. SMAD4 is involved in TGFβ signaling pathways, and has a correlation with increased metastasis in cases where it is mutated or lost, making it of interest as a genetic source of PDA’s lethality. This metastatic dependence on SMAD4 dysfunction was confirmed by the very low rate of lung metastasis seen in mice after tail injection with SMAD4-/- pancreatic cancer cells reconstituted with functional SMAD4 as compared to the LacZ control (Figure a). A meta-analysis of the transcriptional targets of SMAD4 in PDA cell lines revealed hundreds of candidate genes, many of which have previously been implicated in metastasis. In an attempt to more specifically target these genes, an in vivo selection assay was used to find individual genetic “hits” that would increase the metastatic potential of isogenic cell lines dependent on SMAD4 for metastasis suppression (Figure b). Genes that SMAD4 normally upregulates were knocked out via CRISPR/Cas9, while genes that SMAD4 normally downregulates were overexpressed using a barcoded open reading frame (ORF) library. The sgRNA or ORF barcodes most prevalent in the lung metastases thus represent the hits which are genetically capable of increasing the metastatic ability of these cells. The results of this screen will be followed by further investigation of the most promising hits, especially those with previously documented presence in PDA patients or that affect a phenotype logically connected to metastasis. While initial screens were conducted using human cells in murine models, syngeneic murine models will be used to reveal interactions between this metastatic phenotype and immune response. Long-term results could then be used for targeted therapies involving nanoparticles to seek out and deliver therapies to the highly metastatic members of a PDA tumor population even after the cells have spread.