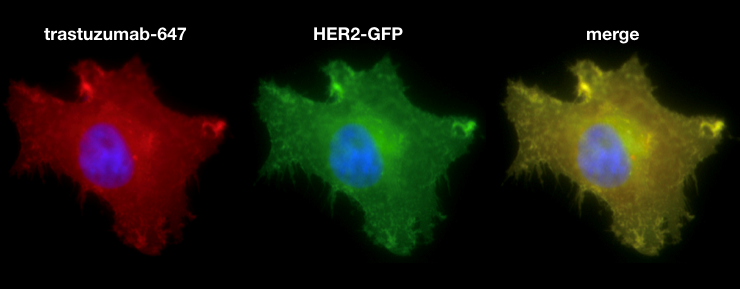
15-30% of breast cancers overexpress the human epidermal growth factor receptor 2 (HER2), a transmembrane receptor tyrosine kinase. HER2 overexpression drives aberrant proliferation and increases the likelihood of metastasis. Current treatment for HER2+ breast cancer relies on targeted therapy, including monoclonal antibodies (mAB) and antibody-drug-conjugates such as trastuzumab and ado-trastuzumab emtansine (T-DM1), respectively. More advanced formulations, including HER2-targeted immunoliposomal nanotherapy (e.g., MM-302 liposomal encapsulated doxorubicin, Merrimack Pharmaceuticals), are being clinically tested but with mixed success. Although HER2 targeting has drastically improved the prognosis of HER2+ breast cancer patients, resistance often develops in advanced disease. Average survival in patients with advanced HER2+ disease is less than 3 years. One hypothesis for treatment failure is inefficient delivery of drugs to tumors. Antibodies are known to have issues fully penetrating to poorly vascularized tumors, and larger drug delivery vehicles such as antibody-liposome conjugates face even greater barriers. The MGH Center for Systems Biology has recently developed new imaging technologies, especially confocal fluorescent microscopy in live animal models of disease (intravital imaging) to closely study drug delivery in solid tumors. This technology has led to the discovery of new “tumor-priming” approaches to improve passive (non-targeted) nanotherapy delivery. My work aims to extend these studies to molecularly-targeted treatments, particularly anti-HER2 antibody and targeted-nanoparticle therapies. I will also explore the efficacy and mechanism of trastuzumab targeted nanoparticles in vivo as a cytotoxic drug carrier. The successful completion of this project will provide deeper insight on barriers to effective HER2 targeting, which will guide the design of future targeting therapies or combination treatment strategies.