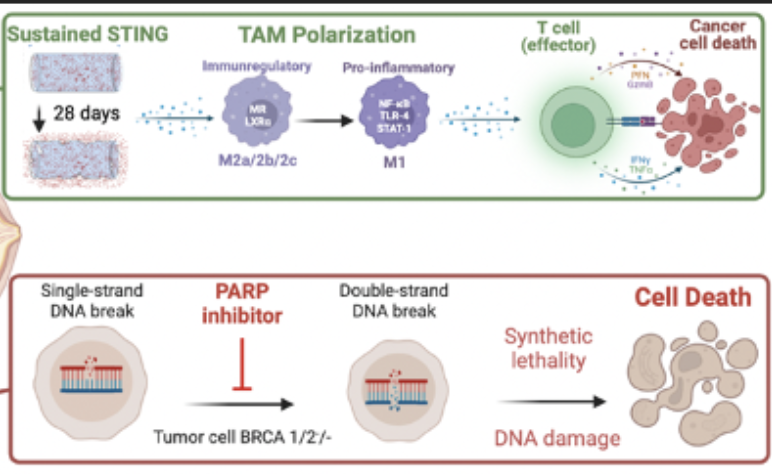
Nanoparticle drug delivery options for breast and ovarian cancers are becoming more effective in treating malignant tumors while decreasing the toxic effects of cancer treatment. This project combines the formulation of ADU-S100 with Talazoparib (TLZ) to test the synergy of the two drugs in treating breast and ovarian cancers and overcoming barriers to drug delivery. TLZ is a drug consisting of PARP inhibitors (PARPi), which are a class of drugs used to treat BRCA-deficient ovarian cancers by interrupting the DNA repair pathway of tumor cells, leading to cell damage and eventually cell death in the tumor. Clinically, STING agonists are administered intratumorally, which require weekly injections and increased patient burden to deliver a high drug load to the tumor but minimize systemic inflammatory responses. Sustained delivery implants are a way to mediate these concerns, and implants made with poly (lactic-co-glycolic) acid (PLGA) loaded with ADU-S100 are being tested to improve the efficacy and reduce toxicity in breast and ovarian cancer. This response has been shown to synergize with the addition of TLZ to overcome challenges with drug resistance and lead to complete cure in breast cancer preclinical models, however delivery of this therapy limits effective translation. The objective of the project is to test the synergy between ADU-S100, a STING agonist, and TLZ when used as an anti-tumor treatment in BRCA-deficient cancer cell lines using a biodegradable sustained delivery implant. In vitro studies will be carried out to assess DNA damage and treatment efficacy of both drugs together and alone in cancer cell lines with BRCA gene deficiency.