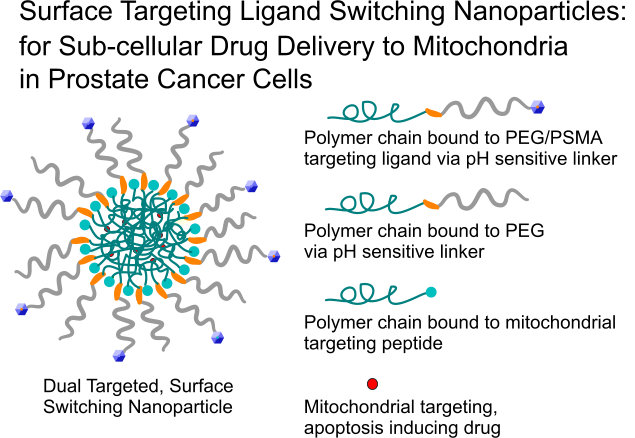
An emerging method for targeting drugs to disease areas is to exploit the local changes that occur due to disease pathology and use these changes as triggers to improve targeting. This is accomplished by developing stimuli-responsive materials that change their physicochemical or drug-release properties upon encountering specific environmental cues, potentially leading to increased drug delivery to diseased tissues. These environmental cues may include low pH, inflammation, the presence of certain unique enzymes, and the reducing environment of endosomes. It may be possible to improve nanoparticle (NP) targeting to sites of disease by causing changes in NP surface properties at the target sites. In addition, the design of nanoparticles that can be internalized by endocytosis and thus release their active drugs inside subcellular organelles can be used to overcome multidrug resistance in cancer cells. In this project we develop surface switching NPs that can target first prostate cancer cells using a small molecule ligand and then the mitochondria specifically following endocytosis and endosomal release using a mitochondrial targeting peptide. This system would then be able to deliver its drug, which promotes pro-apoptosis signaling factors, directly to the mitochondria of the cell and create an efficient anti-cancer treatment.