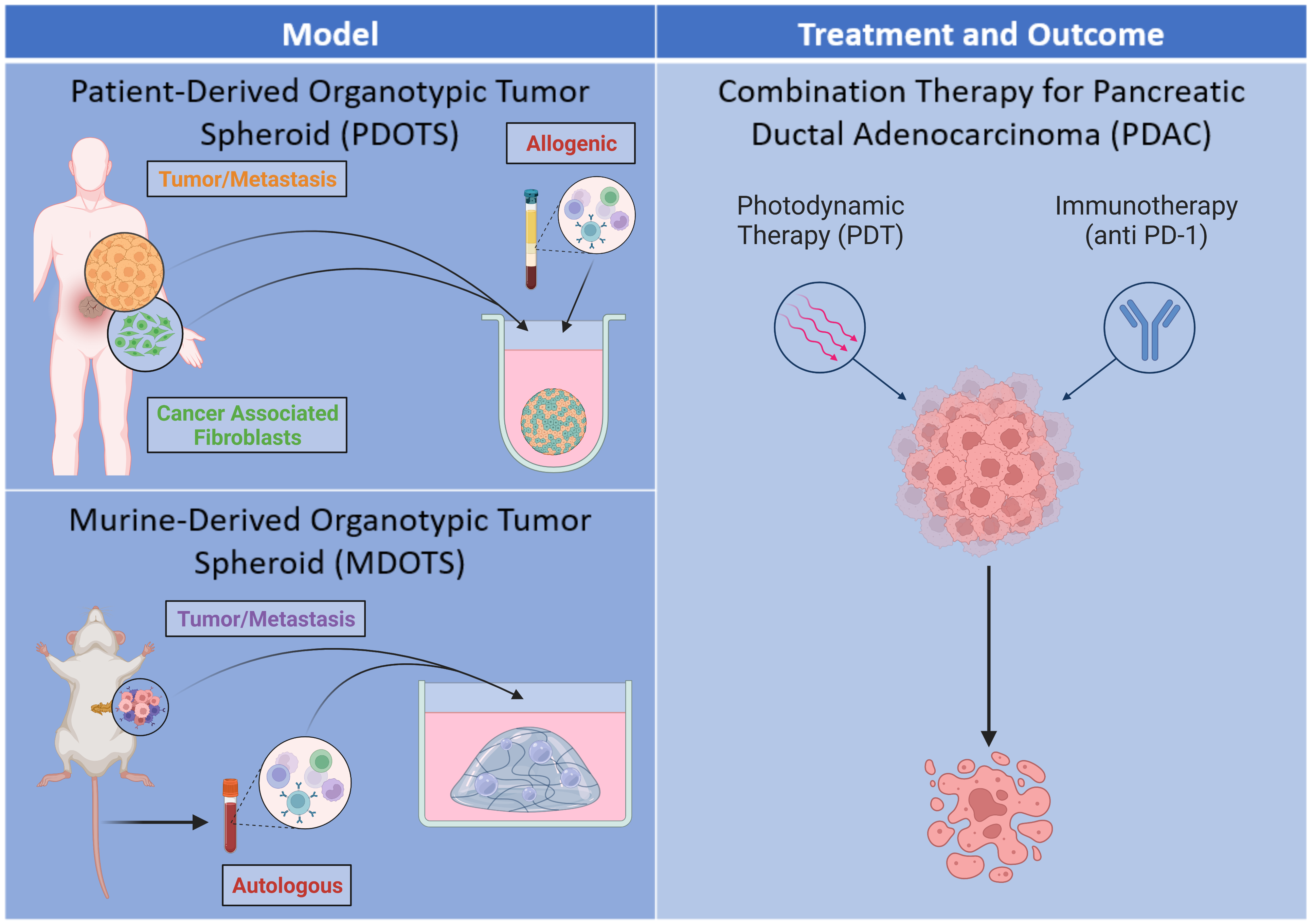
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer subtype characterized by desmoplasia. Desmoplasia is defined as the development of fibrous connective tissue, composed of fibroblasts and extracellular matrix, in response to certain malignancies, including cancer. The desmoplastic response significantly reduces drug distribution and efficacy due to the presence of a dense stroma and contributes to a more hostile tumor microenvironment (TME) — both of which result in poor prognosis and increased tumor metastasis and recurrence in PDAC patients. Photodynamic therapy (PDT) is a potential cancer treatment option in which a nontoxic photosensitizer absorbs light and undergoes excitation to a higher-energy state prior to relaxation to a lower-energy excited triplet state. The excited triplet state of the photosensitizer can transfer energy or electrons to surrounding molecules, resulting in the formation of cytotoxic reactive molecular species, such as singlet oxygen, and inducing cell death. PDT may be leveraged in the resensitization of therapy-resistant cancers, including those unresponsive to immunotherapies. Subtherapeutic PDT, also referred to as photodynamic priming (PDP), transiently alters the TME, increasing permeability to improve drug delivery and producing immune-stimulatory signals such as damage-associated molecular patterns (DAMPs) which induce immunogenic cell death. The release of DAMPs results in the recruitment of antigen-presenting cells which engulf the dying tumor cells and activate cytotoxic T cells responsible for tumor killing. However, the surviving tumor cells may express the PD-1 ligand (PD-L1), which binds to the PD-1 receptor on T cells to suppress anti-tumor immune responses. Anti-PD-1 is an immune checkpoint inhibitor that blocks the PD-1 receptor and enhances anti-tumor immune responses in patients. This project aims to mimic the TME observed in PDAC patients through two distinct in vitro three-dimensional heterogeneous tumor models — the first will be a patient-derived organotypic tumor spheroid (PDOTS) created by coculturing malignant MIA PaCa-2 pancreatic cancer cells with various amounts of pancreatic cancer-associated fibroblasts; the second will be a murine-derived organotypic tumor spheroid (MDOTS) created by isolating pancreatic tumors and the surrounding stroma obtained from orthotopic mouse tumors. Both models will be treated by PDT prior to the addition of peripheral blood mononuclear cells and anti-PD-1 to determine the efficacy of combination treatment in inducing cell death. Ultimately, this project will attempt to develop a tumor model for evaluating the response of PDT and anti-PD-1 combination therapy in PDAC.
CaNCURE Research Presentation: https://youtu.be/VwmfEZI0DWM